Functional features of the associated fungi of bark beetles and the prospect of management of the synergetic infection of insect pests and pathogens
-
摘要:
昆虫作为媒介传播的林木病害在全球范围广泛分布,并造成毁灭性的危害。害虫和病原物对林木形成复合危害,因其发生机理复杂和危害症状隐蔽,以及二者间通过跨界互惠共生提高单个物种的适生度、增强对环境变化的缓冲适应能力和维持特定的生态功能等特点,使得对这类病虫复合危害的控制难度极大。小蠹虫(象甲科Curculionidae 小蠹亚科Scolytinae)与真菌在长期协同进化过程中,形成了稳定的体外共生关系,这些真菌被称为小蠹虫的伴生真菌(associated fungi),其中许多种类能引起严重的森林病害,并与小蠹虫表现出密切的互惠共生关系。本文主要围绕小蠹虫和伴生真菌中了解最深入的长喙壳类真菌形成的伴生体系,从伴生真菌多样性、长喙壳类真菌生态功能和病虫复合危害防控展望3个方面,介绍相关领域的最新研究进展,为认识这类危害的作用机理积累科学基础,并为森林病虫害的有效控制提供新思路。
Abstract:Insect-borne diseases are a class of major forest diseases that occur globally in epidemic proportions. Both insect pests and pathogens form synergetic infection to forest trees, which are extremely difficult to control due to the complexity of their mechanisms of occurrence, the insidious nature of damage symptoms, and mutualistic symbioses between insect pests and pathogens, which increase the fitness of each individual species, enhancing the buffering and adaptive capacity of species to environmental change, and maintaining specific ecological functions. Bark beetles (Curculionidae Scolytinae) and fungi have formed stable ectosymbiosis during the long-term co-evolution process. These fungi are called associated fungi of bark beetles, many of which can cause serious forest diseases and show close mutualistic symbioses with beetles. The present paper focuses primarily on the bark beetle-ophiostomatoid fungi companion system, which is the most well understood associated fungi, and provides an overview of the latest research progress in related fields from three perspectives, namely, the diversity of associated fungi, ecological functions of ophiostomatoid fungi, and prospect of strategies for prevention and control of the synergetic infection of insect pests and pathogens. It is hoped that the findings summarized in this review can aid in building a scientific basis for understanding the mechanisms of action of such damage and offer novel ideas for effective control of forest insect pests and pathogens.
-
气候变暖、生物入侵等在全球范围内加剧,引发了森林病虫害的大范围扩散流行。大面积营造的人工林往往由于树种单一、缺乏冗余结构造就的复杂功能,而在抵御病虫害发生和灾后修复中韧性不足,使得病虫害在各大洲造成景观尺度的巨大危害(Popkin, 2021;Williams et al., 2023)。在中国,松钻蛀类害虫近年来成为了继松材线虫病后,危害和扩散形势最为严峻的林业有害生物,发生面积居高不下,呈分布范围广、虫口基数大的发生格局(刘冰等,2024)。这类害虫成灾除了依靠自身种群迅速增长外,另一个重要原因是携带传播病原菌引起树木枯萎和溃疡病,是典型的害虫和病原对森林的复合危害,如各种小蠹虫和携带传播的长喙壳类病原真菌。新时期,传统的森林保护学迎来了树木医学维度的新发展阶段(田呈明和张星耀,2024),为从全生命体角度审视病虫复合危害提供了契机,对于认识有害生物成灾机理和创新防控新技术切实维护森林健康,有着重要意义。
小蠹虫隶属于鞘翅目Coleoptera象甲科Curculionidae的小蠹亚科Scolytinae和长蠹亚科Platypodinae,根据生活习性又可分为树皮小蠹(bark beetle)和食菌小蠹(ambrosia beetle)。小蠹虫物种多样性丰富,对促进生态系统的物质循环具有积极作用(Raffa et al., 2015)。然而,在气候变化背景下,小蠹虫种群的暴发已造成北半球大范围针叶林的死亡,导致的经济损失堪比飓风和森林火灾(Grégoire and Evans, 2004)。其中,造成严重危害的类群主要包括齿小蠹属Ips spp.、大小蠹属Dendroctonus spp.和切梢小蠹属Tomicus spp.等种类。
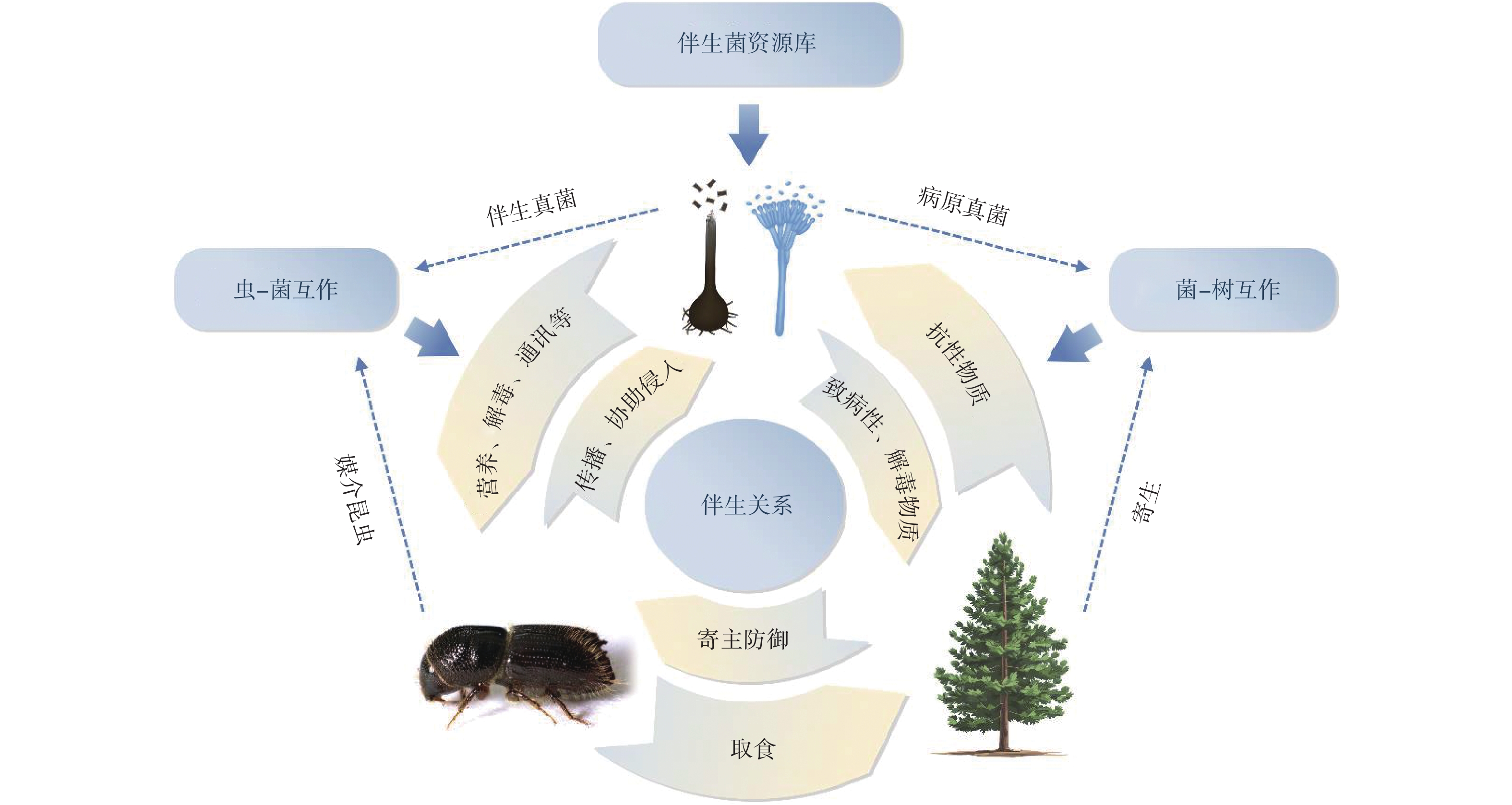
小蠹虫在长期进化中与真菌形成稳定的体外共生关系,二者在森林生态系统中生态位重叠,发展出相互依存的互惠共生关系,这些真菌被称为小蠹虫的伴生真菌(associated fungi)。寄主树木−小蠹虫−伴生真菌之间的互作关系十分复杂,包括互惠共生、寄生和拮抗等(图1)。从寄主角度而言,其会利用抗性物质、物理屏障等防御机制应对小蠹虫的取食和伴生真菌的致病性;从小蠹虫角度而言,蛀干害虫利用其成虫钻蛀入树体内的习性可携带传播伴生真菌,并且协助其侵入寄主组织;伴生真菌则可通过提供营养、解毒(或克服寄主防御)和调控小蠹虫种群聚集等方式使小蠹虫获益。同时,伴生真菌又可作为病虫复合危害防控的资源库。本文以小蠹虫伴生真菌多样性为切入点,梳理长喙壳类真菌生态功能,展望虫菌复合危害防控前景。
1. 小蠹虫伴生真菌多样性
随着多种小蠹虫类群造成的危害周期性暴发成灾,其伴生真菌区系也受到研究者的关注,并逐步得到解析,呈现出丰富的物种和区系多样性特征。越来越多的证据表明,小蠹虫与伴生真菌间存在着协同进化,“特异性伴生”以及伴随的“协同成种”是其中的一个重要特征。
1.1 物种多样性
与小蠹虫伴生的真菌种类主要分布在子囊菌门Ascomycota和担子菌门Basidiomycota,其中子囊菌门的长喙壳类真菌(ophiostomatoid fungi)是小蠹虫伴生真菌中最主要的类群,得到了系统和广泛的研究。长喙壳类真菌是形态和生态特征相似,系统发育相距较远的一类真菌的总称(Kirisits, 2004;de Beer et al., 2013)。在长期进化过程中,该类真菌发育形成了包括具有长喙状的子囊壳、延长柄的分生孢子梗或孢子梗束,以及各自顶端的黏性子囊孢子团和分生孢子团等的特殊形态构造。这类构造十分利于昆虫的携带和传播(Wingfield et al., 1993)。长喙壳类真菌统指子囊菌门的2个系统分支:肉座菌亚纲Hypocreomycetidae的微囊目Microascales的部分类群和粪壳菌亚纲Sordariomycetidae的长喙壳目Ophiostomatales的所有类群,包括微囊目的3科12属和长喙壳目的1科20属,合计500余种(de Beer et al., 2013, 2014, 2022)。在中国,自与切梢小蠹伴生的云南细帚霉Leptographium yunnanense X.D. Zhou, K. Jacobs, M.J. Wingf. & M. Morelet(Zhou et al., 2000)被发现以来,已有10属(包括长喙壳目的Ceratocystiopsis、Esteya、Graphilbum、Grosmannia、Leptographium、Masuyamyces、Ophiostoma、Sporothrix和微囊目的Endoconidiophora、Graphium)167种小蠹虫伴生长喙壳类真菌陆续被报道(Wang et al., 2024b, c)。其中大多数物种属于长喙壳目的长喙壳属Ophiostoma spp.和细帚霉属Leptographium spp.,分别为67种和44种(Wang et al., 2024b, c)。
隶属子囊菌门肉座菌目Hypocreales的Geosmithia属也是一类广泛分布但长期被忽视的小蠹虫伴生真菌(Pepori et al., 2018; Kolařík and Hulcr, 2023)。该属具有相对较高的系统发育多样性,超过68个系统发育种,其中33个物种已被正式描述(Kolařík and Hulcr, 2023;梁玲瑜等,2024)。目前,中国报道了13个物种,远低于欧洲及地中海周边的36种和北美洲的47种。此外,子囊菌类和担子菌类的酵母菌也是小蠹虫伴生真菌中的优势类群(Lim et al., 2005;Giordano et al., 2013;Davis, 2015),但有关其系统发育和分类的资料十分有限和零散,本文不对其多样性进行讨论。
自然界可培养微生物种类仅占全部微生物区系的极小一部分,绝大多数为不可培养微生物。与昆虫伴生的微生物种类绝大多数也属于不可培养类型,在伴生关系中同样起着重要作用。宏基因组学等新一代测序技术的应用极大推动了环境微生物群落的挖掘。利用高通量测序分析小蠹虫体表伴生真菌,在验证了可培养区系物种组成的多样性和丰度的基础上,极大地拓展了对群落组成多样性的认识(Kostovcik et al., 2015),包括子囊菌、担子菌、球囊菌、芽枝霉、壶菌和接合菌等6个门142个科的416个种和948个OTUs与切梢小蠹伴生(Wang et al., 2019)。
1.2 区系多样性
解析长喙壳类真菌的区系组成对于揭示小蠹虫伴生真菌多样性的形成机制及虫菌伴生关系具有重要意义,体现在不同地区、不同小蠹和不同寄主3个方面中真菌类群的发生规律。由于大多数不同种类小蠹虫的地理分布与寄主范围并不重叠,导致难以控制单一变量进行比较(Wang et al., 2024b)。因此,具体哪个因素对长喙壳类真菌的区系组成起主要作用仍不明确。
目前,中国已知3种大小蠹的伴生长喙壳类真菌种类,分别为红脂大小蠹Dendroctonus valens LeConte的15种(Lu et al., 2009a, b;Marincowitz et al., 2020)、云杉大小蠹Dendroctonus micans Kugelann的14种(Yin et al., 2016;Chang et al., 2020;Wang et al., 2021)和华山松大小蠹Dendroctonus armandii Tsai et Li的7种(Wang et al., 2022)。尽管三者都以松科植物为寄主,但是它们的伴生真菌区系完全不同,没有共享物种。从地理因素分析,国内的红脂大小蠹和云杉大小蠹与美国、欧洲的同种小蠹虫伴生长喙壳类真菌区系也几乎不重叠(Lieutier et al., 1992; Marincowitz et al., 2020)。对分布于中国东北、西北、青藏高原及其周边地区针叶林中10种齿小蠹伴生真菌的系统研究(Wang et al., 2024b),为区系多样性比较提供了理想模式。不同虫种、寄主和地理分布均影响了齿小蠹伴生真菌区系丰度和多样性的组成,且同一影响因素下,小蠹虫伴生真菌区系重叠种类均小于5种(Wang et al., 2024b)。利用宏基因组的高通量测序方法分析云南切梢小蠹Tomicus yunnanensis Kirkendall & Faccoli、横坑切梢小蠹Tomicus minor Hartig和短毛切梢小蠹Tomicus brevipilosus(Eggers)体表伴生真菌区系组成,结果表明,地理分布和寄主对小蠹虫体表真菌区系组成影响较大,而切梢小蠹种类对其影响并不显著(Wang et al., 2019)。
1.3 种特异性伴生关系
近年来通过分子序列的系统发育分析等方法,不断揭示出小蠹虫伴生真菌隐存种和种下分化的存在。越来越多的研究表明,小蠹虫与伴生真菌间存在着协同进化,一个重要特征便是“特异性伴生”(Six, 2012),以及往往同时存在着的“协同成种”。这种特异性伴生的现象在大小蠹属内得到了反复印证(Six and Paine, 1997;Alamouti et al., 2011;Roe et al., 2011)。西松大小蠹Dendroctonus brevicomis Le Conte和其他小蠹常混生在一起,但仍然在大范围内不同季节的变换中,与特定种类的真菌存在着稳定的种间特异性伴生。进一步研究发现双方种下协同趋异分化的现象:优势伴生真菌Entomocorticium sp. B有不同单体型的分化,形成了2个不同的地理变异种群,其宿主小蠹也存在2个地理亚种(Bracewell and Six, 2014)。然而,只有与小蠹虫宿主来源同一个地区的伴生真菌,在实验室控制条件下与小蠹虫形成伴生关系(在小蠹虫储菌器内发现),而非同一来源的伴生真菌个体无法与小蠹虫宿主形成伴生关系,表明小蠹虫对特定种类伴生真菌的选择性,即种或基因型的特异性伴生(Bracewell and Six, 2015)。对山松大小蠹Dendroctonus ponderosae Hopkins伴生真菌Leptographium clavigerum(H.P. Upadhyay)T. C. Harr., Six & McNew的67个基因组区域的碱基差异进行筛选,开发出15个蛋白质编码基因片段进行系统发育分析,发现L. clavigerum种下分化出2个明显的种群(Lee et al., 2007;Alamouti et al., 2011;Roe et al., 2011),验证了种间特异性伴生的关系。
在欧亚大陆广泛分布并造成严重危害的齿小蠹中,也发现与先锋长喙壳类真菌存在种间特异性伴生现象,包括欧洲云杉Picea abies(L.)H. Karst.−云杉八齿小蠹Ips typographus Linaaeus−Endoconidiophora polonica(Siemaszko)Z. W. de Beer, T. A. Duong & M. J. Wingf、欧洲落叶松Larix deciduas Mill−欧洲落叶松八齿小蠹Ips cembrae(Heer)−Endoconidiophora laricicola(Redfern & Minter)Z. W. de Beer, T. A. Duong & M. J. Wingf和日本落叶松Larix kaempferi(Lamb.)Carr−亚洲落叶松八齿小蠹Ips subelongatus Matschulsky−Endoconidiophora fujiensis M. J. Wingf., Yamaoka & M. Marín) Z.W. de Beer, T. A. Duong & M. J. Wingf的“寄主−害虫−病原菌”的特异性伴生(Harrington et al., 2002;Marin et al., 2005;孟贤静等,2015)。这3种齿小蠹形态和遗传距离都极为接近,与此类似的是,各自稳定伴生的真菌E. polonica、E. laricicola和E. fujiensis在形态上也几乎无法区分,借助多基因序列的系统发育分析才将它们分为不同种。这种特异性伴生关系也延展到了种下分化。亚洲的云杉八齿小蠹种群发生分化,形成有别于欧洲种群的一个亚种(Stauffer and Lakatos, 2000;Sallé et al., 2007),平行地,二者的主要伴生真菌E. polonica 的遗传分析也表明两个地区的种群存在显著的遗传差异(Marin et al., 2009),即欧洲云杉−云杉八齿小蠹−E. polonica欧洲种群和日本云杉P. jezoensis−云杉八齿小蠹日本亚种I. typographus f. japonicus Niijima−E. polonica日本种群协同分化现象的发生。在中国落叶松八齿小蠹的伴生真菌E. fujiensis在宿主小蠹发生种下分化(Song et al., 2011)的同时,也存在丰富的种下分化特征(孟贤静等,2015)。最近的研究发现2组新的伴生组合,即西伯利亚落叶松Larix sibirica Ldb.−亚洲落叶松八齿小蠹−E. laricicola和青海云杉Picea crassifolia Kom.−云杉大小蠹−E. laricicola,表明E. laricicola可能发生了媒介昆虫的跨物种传播及对非落叶松寄主的危害(Wang et al., 2021, 2024b)。
伴生真菌区系发生规律也与其媒介齿小蠹的遗传分化和种群形成有明显关系。通过对欧洲、日本、中国东北和新疆地区的云杉八齿小蠹伴生真菌区系比较发现,4个地区呈现极少数共有、绝大多数特有的特点(Paciura et al., 2010;Chang et al., 2019;Wang et al., 2024b),恰好与云杉八齿小蠹的4个遗传群体匹配(Mayer et al., 2015;王正等, 2021)。在中国,已报道的118种齿小蠹伴生长喙壳类真菌中的96种是与它们各自的齿小蠹媒介特异性伴生的(Wang et al., 2024b)。有趣的是,姊妹种东喜马拉雅云杉齿小蠹Ips schmutzenhoferi Holzschuh和西藏重齿小蠹Ips stebbingi Strohmever的伴生真菌同样是姊妹种。在欧洲,齿小蠹属昆虫的2种主要伴生真菌E. polonica 和E. laricicola都是强致病菌,具有明显的寄主分化特征,接种试验证明只能在各自的寄主(欧洲云杉或欧洲落叶松)上造成显著的病斑(Harrington et al., 2002)。野外接种试验也表明,E. fujiensis的致病性在不同落叶松上存在着显著的寄主分化特征(Wang et al., 2020)。这些遗传和致病力差异的发现为进一步揭示种间特异性伴生规律提供了有力的证据。
2. 长喙壳类真菌生态功能
小蠹虫与伴生真菌间存在着密切的共生关系,这种共生因昆虫宿主的种类繁多、危害严重和共生真菌的严重致病性,以及受气候变化等因素影响显著,多年来一直是共生关系中的研究热点(Guerrero et al., 2013)。小蠹虫可以从伴生长喙壳类真菌受益的方式,包括利用真菌病原物的致病性,直接引起寄主病害,甚至死亡,以协同克服寄主抗性;另外,长喙壳类真菌还会通过为小蠹虫生长发育供给营养、解毒林木寄主防御性化合物、产生信息素增强小蠹虫种群聚集以成功侵入寄主,以及拮抗有害微生物等多种方式,为二者的共生发挥作用。
2.1 引起植物病害
长期以来,伴生真菌的致病性被认为是小蠹虫−真菌共生关系中的一个重要元素,是小蠹虫从共生关系中获利的主要特征。伴生真菌的强致病性或群体致病性能够协同小蠹虫克服、消耗寄主抗性,使小蠹虫以更低的种群密度成功侵入寄主并实现定殖(Lieutier et al., 2009)。长喙壳类真菌作为病原菌造成多种毁灭性的森林病害,最著名的是榆树枯萎病(Dutch elm disease, DED),病原菌榆长喙壳Ophiostoma ulmi (Buisman) Nannf和新榆长喙壳O. novo-ulmi Brasier在榆小蠹Scolytus spp.取食健康榆树枝条时,被携带至新的寄主,引起榆树输导组织堵塞,整个枝条或者树干在短时间内迅速枯萎死亡。该病害于20世纪初和20世纪40年代在欧美连续2次大流行,给当地榆树造成灭顶之灾(Brasier et al., 2021)。近几年,这2种病原菌相继在日本和韩国被发现(Masuya et al., 2010;Miyamoto et al., 2019;Lee et al., 2022),榆树枯萎病病原菌传入中国的风险陡增。除此之外,还有多种长喙壳类真菌引起的林木病害,症状包括蓝变、枯萎、溃疡、腐烂、黑根等(表1)。观察发现,与致病性真菌伴生很可能是某些侵入能力强的小蠹虫的一个重要特征,甚至是小蠹虫侵入寄主的一个先决条件(Christiansen et al., 1987)。
表 1 长喙壳类真菌引起的主要病害Table 1. Diseases caused by ophiostomatoid fungi病原
Fungus分布
Distribution寄主
Host病害
Disease参考文献
ReferenceBretziella fagacearum 北美洲 栎树Quercus spp. 栎树枯萎病 de Beer et al., 2017 Ceratocystis acaciivora 南非 金合欢Acacia mearnsii 枯萎 Tarigan et al., 2011 C. albofundus 南非 金合欢A. mearnsii 枯萎
WiltRoux et al., 2007; Nasution et al., 2019 C. fimbriata 热带、亚热带和温带 多种农作物
Variety of agricultural crops腐烂、溃疡、枯萎 Baker et al., 2003 C. fimbriata 非洲中部、南部、
南美、亚洲桉树Eucalaptus spp. 枯萎 Roux et al., 2000; Roux and
Wingfield, 2009C. fimbriata f. platani 欧洲、北美洲 悬铃木Platanus spp. 溃疡、枯萎 Tsopelas and Angelopoulos, 2018 C. hulioh
C. lukuohia北美洲 铁心木
Metrosideros polymorpha枯萎 Barnes et al., 2018 C. manginecans 阿曼、巴基斯坦 芒果、金合欢
Mango, A. mearnsii枯萎 Van Wyk et al., 2007 C. pirilliformis 南非、澳大利亚 桉树Eucalaptus spp. 枯萎 Roux et al., 2004 C. tsitskammensis 南非 金合欢A. mearnsii 枯萎 Van der colff et al., 2017 Chalara australis 澳大利亚 假山毛榉Nothofagus spp. 枯萎 Kile and Walker, 1987 Davidsoniella virescens 北美洲 糖槭Acer sacharum 枯萎、腐烂 Bal et al., 2013 Endoconidiophora fujiensis 欧洲、日本、中国 落叶松Larix spp. 溃疡 Yamaoka et al., 1998; Liu et al., 2024 E. laricicola 欧洲、日本、中国 落叶松Larix spp. 溃疡 Yamaoka et al., 1998; 刘亚, 2024 E. polonica 欧洲、日本、中国 云杉Picea spp. 溃疡 Yamaoka et al., 2000;
Hlásny et al., 2021; 刘亚, 2024E. rufipenni 北美洲 云杉Picea spp. 溃疡 Solheim and Safranyik, 1997 Leptographium clavigerum 北美洲 美国黑松Pinus contorta 溃疡 DiGuistini et al., 2011 L. procerum 北美洲、欧洲、
新西兰、中国松树Pinus spp. 根病 Lu et al., 2009b L. qinlingense 中国 华山松P. armandii 溃疡 Wang et al., 2024a L. serpens 欧洲、南非 松树Pinus spp. 根病 Matusick et al., 2012 L. wageneri 北美洲 针叶树Conifers 黑根病 Cobb, 1988 L. wingfieldii 欧洲 松树Pinus spp. 溃疡 Hausner et al., 2005 L. yunnanense 中国 云南松P. yunnanensis 溃疡 Pan et al., 2018; 王慧敏, 2019 Ophiostoma dryocoetidis 北美洲 高山冷杉Abies lasiocarpa 溃疡 Lalande et al., 2020 O. minus 欧洲、北美洲 松树Pinus spp. 溃疡 Gorton and Webber, 2000 O. narcissus 北美洲、欧洲、新西兰 水仙花Narcissus spp. 黑根病 Davies et al., 1998 O. ulmi
O. novo-ulmi北美洲、欧洲、
亚洲、新西兰榆树Ulmus spp. 榆树枯萎病 Potter et al., 2011 Raffaelea lauricola 美国东南部 樟科Lauraceae 枯萎 Harrington et al., 2008;
Rodrigues et al., 2020Sporothrix schenkii
S. brasiliensis
S. globosa世界性的 人类Humans 孢子丝菌病 Rodrigues et al., 2013, 2020 Thielaviopsis paradoxa 热带、亚热带 多种农作物
Variety of agricultural crops根腐、果腐 Pinho et al., 2013 与小蠹虫伴生的长喙壳类真菌具有多样化的致病力水平,除能直接致死健康寄主外(Yamaoka et al., 1998),还能够消耗寄主抗性物质,协助小蠹虫以较低水平入侵、定殖健康寄主。红脂大小蠹成功入侵中国,与其伴生真菌长梗细帚霉Leptographium procerum (W.B. Kendr.) M.J. Wingf具有独特的基因型、表现出更高的寄主致病性、能够降低寄主油松抗性和诱导寄主产生红脂大小蠹聚集信息素3-蒈烯等有关,而3-蒈烯是红脂大小蠹最有效的引诱剂,因而促进了红脂大小蠹向寄主聚集,提高了红脂大小蠹入侵过程中的适应性(Lu et al., 2010)。基于上述结论,研究者提出了返入侵假说:在原产地的次期性害虫−伴生菌组合,在新的入侵地发生变异后,如再返回到原产地,也有可能造成严重的生物灾害(Lu et al., 2011)。引起榆树枯萎病的新榆长喙壳也能够上调榆树倍半萜释放量,吸引更多的媒介昆虫榆小蠹聚集(McLeod, 2005)。
长喙壳类真菌种类繁多,除危害林木外还是多种农作物病原菌,甚至对人类或动物健康也有相当大的影响。长喙壳类真菌引起多种农作物的腐烂病、溃疡病和枯萎病,如Ceratocystis fimbriata Ellis & Halst.引起甘薯黑斑病(Halsted, 1890;Baker et al., 2003),Thielaviopsis paradoxa(De Seynes Höhn.)引起香蕉Musa nana Lour.、杨桃Averrhoa carambola L.、椰子Cocos nucifera L.和菠萝Ananas comosus (Linn.) Merr.等热带水果和作物的根腐病及果腐病(Ploetz, 2003; Pinho et al., 2013;de Beer et al., 2014)。而Sporothrix属的种类引起人类或动物孢子丝菌病(Sporotrichosis),通常入侵人体或动物的皮肤和黏膜。
2.2 营养供给
木材本身营养贫乏,真菌成为生活在其中的昆虫的重要营养来源(Koski et al., 2024)。小蠹虫完全或部分依赖于共生真菌提供食物以完成其生活史,真菌共生体的这种作用被称作小蠹虫的“根系系统”或“外部胃”(Hulcr and Dunn, 2011)。材小蠹又被称为“食菌小蠹”,幼虫在木质部内发育,木质部由难以消化利用的木质素构成,小蠹虫个体发育完全依靠取食坑道内生长的真菌——“栽培作物”。针叶树韧皮部也是一个营养相对贫乏的环境(Scriber and Slansky, 1981),其N和P含量分别比昆虫所需的低数十倍甚至数千倍(Fagan et al., 2002;Filipiak and Weiner, 2014)。这种情况下,真菌为小蠹虫的生长发育提供了必需的氮素、氨基酸、碳水化合物和甾醇等(Ayres et al., 2000;Bentz and Six, 2006)。真菌的生长也使植物基质更适合小蠹虫取食、消化和吸收(Dowd, 1992)。食菌小蠹以及同时取食木质部或韧皮部和真菌的树皮小蠹能够更有效地获取营养,1 a可以完成1个或者数个发育历期,而在同样栖境中没有与真菌共生的吉丁虫Buprestidae、天牛Cerambycidae等鞘翅目昆虫,却往往需要数年才能完成1个发育历期(Harrington, 2005)。
伴生真菌能够改变小蠹虫的取食策略。没有真菌可以取食的小蠹虫为了获取足够的营养用于发育,往往需要比有真菌伴生的小蠹虫构筑更长的坑道,并通过大量取食才能弥补营养不足。室内控制试验下,小蠹虫幼虫主要集中在被真菌定殖的树皮部分,伴生真菌能够将边材内的氮素吸取并转运到韧皮部供小蠹虫幼虫取食。布满伴生真菌的小蠹虫坑道内氮素含量上升40%,能够显著增大小蠹虫个体(Ayres et al., 2000;Bleiker and Six, 2009)。个体尺寸被认为与小蠹虫存活、繁殖、信息素产生和扩散有密切关系。然而,不同真菌在小蠹虫营养供给上也存在差异,山松大小蠹伴生真菌L. clavigerum比Ophiostoma montium (Rumbold)Arx能够聚集更多的氮素(Cook et al., 2010),而这种差异可能也解释了为何携带L. clavigerum的山松大小蠹比携带O. montium的山松大小蠹发育的更好且存活率更高(Goodsman et al., 2012;Bleiker and Six, 2014)。
红脂大小蠹与伴生菌的互利共生提高了其入侵过程中的适应性,碳水化合物在多营养级间不同的分配模式起到关键作用,氨是调节分配的关键挥发物(Zhou et al., 2017)。氨气诱导了伴生真菌L. procerum葡萄糖淀粉酶AMYG基因调控的淀粉代谢通路,将韧皮部中丰富的淀粉转化为营养价值更高的葡萄糖,补偿了真菌碳水化合物的消耗,维持了“红脂大小蠹−伴生真菌−细菌−寄主油松”跨四界互惠共生体的稳定(Liu et al., 2020)。
2.3 解毒寄主化学成分
小蠹虫侵入寄主的不同阶段受到寄主不同化学成分乃至浓度变化的显著影响(Wallin and Raffa, 2000)。针叶树通常分泌高浓度的萜烯类与酚类化合物抵御小蠹虫及其伴生真菌的定殖(Franceschi et al., 2005;Keeling and Bohlmann, 2006),而伴生真菌表现出的解毒寄主防御性物质,一定程度上保证了小蠹虫的成功入侵。真菌通过自身丰富的酶库,降解寄主植物富含防御性物质的木质纤维素组织,解除萜烯类、生物碱和酚类等对宿主小蠹虫的毒性(Wadke et al., 2016;Itoh et al., 2018;Zhao et al., 2019)。在消耗寄主抗性、协助小蠹虫成功定殖的同时,将这些物质转化为小蠹虫信息素或可被小蠹虫直接消化吸收的成分。D-松醇对红脂大小蠹具有拒食效应,不利于其生长发育,但对其伴生真菌的生长发育具有促进作用。红脂大小蠹利用伴生真菌L. procerum作为其体外降解系统帮助其降解寄主油松中的碳源D-松醇,协助自身入侵寄主与暴发成灾(Liu et al., 2022b)。云杉八齿小蠹和十二齿小蠹Ips sexdentatus Boerner的伴生真菌E. polonica和Ophiostoma brunneo-ciliatum Math.-Käärik均可以降低寄主酚类物质对小蠹虫入侵的抗性(Lieutier et al., 1996;Hammerbacher et al., 2013;Wadke et al., 2016)。山松大小蠹伴生真菌L. clavigerum和O. montium能够富集表达解毒代谢寄主抗性物质的功能基因,可将寄主萜烯类化合物用作碳源供自身生长(Diguistini et al., 2011;Liu et al., 2021)。
青藏高原特有种光臀八齿小蠹Ips nitidus Eggers偏好选择Ophiostoma bicolor R. W. Davidson & D. E. Wells定殖了的树皮基质取食和钻蛀。基因组和转录组的联合分析发现,光臀八齿小蠹取食优势伴生真菌O. bicolor定殖的针叶树树皮基质后,与直接取食没有真菌定殖的树皮基质相比,其解毒相关基因下调表达、高海拔低氧适应相关基因上调表达,显示伴生真菌协助昆虫宿主解毒寄主防御性化合物(Wang et al., 2023),提高了小蠹虫宿主的生态适应性。
2.4 产生小蠹虫信息素
小蠹虫能够产生聚集信息素实现个体之间的高效化学通讯,以达到成功定殖和建立种群的目的。研究表明,长喙壳类真菌在人工培养条件下,可以产生多种挥发性有机化合物,包括醇类、醛类、酯类、酮类、萜类和芳香族化合物等(Morath et al., 2012;Macias-Rodriguez et al., 2015)。这些化合物不仅能够通过促进或抑制真菌的生长和繁殖来影响真菌间的竞争(Cale et al., 2016),还可以作为跨界通信信号(Davis et al., 2013;Davis and Landolt, 2013;Schulz-Bohm et al., 2017)影响小蠹虫行为,吸引小蠹虫定向移动,帮助小蠹虫寻找食物和栖息地,有助于它们的生殖和群体稳定性(Koski et al., 2024),形成二者化学信息素的跨界趋同进化(Zhao et al., 2019)。与云杉八齿小蠹共生的Grosmannia europhioides(E. F. Wright & Cain)Zipfel, Z. W. de Beer & M. J. Wingf可以利用葡萄糖作为碳源,合成其聚集信息素的主要成分2-甲基丁烯醇MB(Zhao et al., 2015)。山松大小蠹伴生真菌L. clavigerum和Ips pini (Say)伴生真菌Ophiostoma ips(Rumbold)Nannf 能够将(-)反式马鞭草烯醇转化为(-)-马鞭草酮(Cale et al., 2019),减少树皮小蠹之间的种内和种间竞争,表明长喙壳类真菌在小蠹虫系统的化学生态中发挥重要作用。
2.5 拮抗有害微生物保护小蠹虫
长喙壳类真菌被证明能够产生乙醇、萜烯等次级代谢产物,拮抗环境中的有害微生物,为小蠹虫提供保护(Davis, 2015;Flórez et al., 2015;Kandasamy et al., 2019)。乙醇是许多小蠹虫引诱剂的主要成分,寄主衰弱木和诱饵木产生乙醇,从而引诱小蠹虫的攻击。最新研究表明,小蠹虫还利用乙醇提高食物产量,保证成功定殖:乙醇可以促进光滑足距小蠹Xylosandrus germanus(Blandford)伴生长喙壳类真菌Ambrosiella grosmanniae C. Mayers, McNew & T. C. Harr、Ambrosiella roeperi T. C. Harr. & McNew和Raffaelea canadensis L. R. Batre的生长,同时能够抑制坑道内的有害伴生真菌Aspergillus 和Penicillium(Ranger et al., 2018)。红翅大小蠹Dendroctonus rufipennis (Kirby)伴生真菌Leptographium abietinum (Peck) M.J. Wingf可以显著抑制白僵菌Beauveria bassiana (Bals.-Criy) Vuill的生长(Davis et al., 2019)。南部松大小蠹有益伴生真菌Entomocorticium sp. A和Ophiostoma ranaculosum(J. R. Bridges & T. J. Perry)Georg Hausner, J. Reid & Klassen通过竞争抑制了对其不利的伴生真菌Ophiostoma minus(Hedgc.)Syd. & P. Syd的生长(Hofstetter et al., 2005)。西松大小蠹共生的酵母菌Ogataea pini(Holst)Y. Yamada, M. Matsuda, K. Maeda & Mikata可以产生乙醇、二硫化碳和Δ-3-蒈烯,这些挥发物可以明显促进伴生真菌虫生伏革菌Entomocorticium sp.的生长,并抑制昆虫病原真菌白僵菌的生长(Davis et al., 2011)。
3. 病虫复合危害防控展望
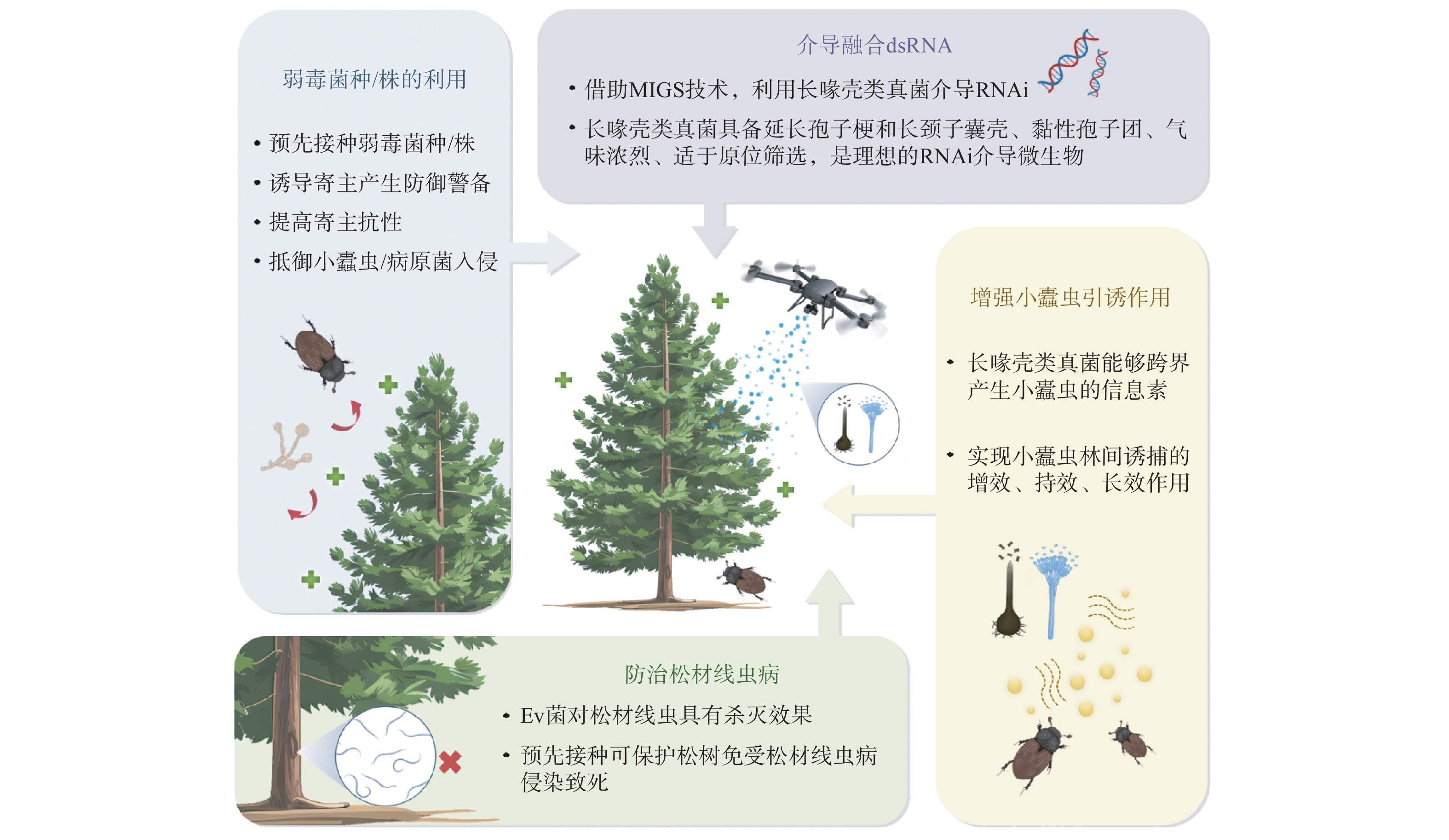
尽管上述研究表明长喙壳类等小蠹虫伴生真菌在协助小蠹虫和自身对寄主的危害中表现出重要作用,但丰富的长喙壳类真菌资源及其复杂的功能特征,也从另外一个方面展现出作为控制小蠹虫等钻蛀类害虫及其传播病害的一个重要潜在途径(图2)。
3.1 长喙壳类真菌弱毒菌种、弱毒菌株的利用
小蠹虫坑道栖居和体表携带多种微生物,这些微生物和昆虫宿主间有着复杂的共生关系,除了互惠共生外,也包括拮抗和偏利共生等,例如南部松大小蠹中的O. minus与小蠹虫存在着拮抗关系(Hofstetter et al., 2006),红脂大小蠹伴生真菌中也存在这样的拮抗关系(Shi et al., 2012)。这些拮抗伴生真菌具有对小蠹虫的生防潜力。
利用和发挥树木自身抗性,是实现森林病虫害持续有效控制的一个根本途径(Jamil et al., 2022)。不同类型长喙壳类真菌致病性具有明显差异,先锋种具有强致病性,引起寄主抗性的长期上调表达,而优势种具有弱致病性,引起寄主短暂且温和的抗性升高(Liu et al., 2024)。将先锋种E. polonica接种到优势种O. bicolor预处理过的红皮云杉上可减小病斑,使蓝变颜色变浅并向木质部内延伸缩小,说明预处理提高了寄主抗性(Liu et al., 2022a)。利用弱毒菌种诱导寄主产生防御警备的绿色疫苗接种方案,产生的抗病效果在落叶松和云杉中得到了相互验证(Wang et al., 2024a)。长喙壳类真菌弱致病菌Ophiostoma canum(Münch)Syd. & P. Syd.诱导提高苏格兰松抗性,抵抗Leptographium wingfieldii M. Morelet的大面积入侵(Krokene et al., 2000)。这一现象解释了小蠹虫伴生真菌群落的演替规律,先锋种主要发生在小蠹虫危害盛期,而优势种则主要发生在小蠹虫种群平稳、有虫不成灾的时期(Solheim, 1992, 1993;Lu et al., 2009b)。
欧洲对板栗疫病的防治是利用弱毒菌株防治森林病害的典型例子。弱毒菌株中的dsRNA真菌病毒CHV-1通过营养亲和传递到强致病力菌株中,减弱病原菌毒力,起到良好防治效果(Rigling and Prospero, 2018)。弱毒菌种O. bicolor中也发现了独特的单链正义RNA病毒ObEV1(Zhu et al., 2022),是否也同板栗疫病弱毒菌株中的真菌病毒一样起到拮抗病原菌的作用,甚至发挥诱导寄主产生系统抗性的作用,将是下一步研究的重点。此外,对于榆树枯萎病,欧美地区多年以来使用的一种已注册的生防菌剂,展现出良好的防治效果(Postma and Geijn, 2016)。维管束病害的病原大丽轮枝菌(Verticillium)的白化非致病性菌株,利用其适合在树木导管系统定殖和生存的特点,通过激发寄主抗性,起到保护树木的作用。长喙壳类真菌白化菌株也被证明可以有效拮抗小蠹虫伴生的2种互惠共生真菌,起到降低小蠹虫危害和控制木材变色的作用(Klepzig et al., 1998),并被开发成商业菌株(Cartapin-97),广泛用于造纸用材的前期处理。
3.2 长喙壳类真菌介导的融合(fusion)dsRNA作用于小蠹虫、病原菌
作为农药史上的第三次技术革命,RNAi生物农药正在对森林病虫害防控产生深刻影响(Singewar and Fladung, 2023)。目前,多项研究表明,基于dsRNA的森林保护产品因其具有种间特异性、没有非标靶效应以及能够改善整个森林健康等优点,展示出广阔的应用前景(Kyre et al., 2019, 2020;Hollowell and Rieske, 2022;张苏芳等,2022)。然而,在树木和森林中使用RNAi技术,不像在农业中易于推广。如何开发新型RNAi介导手段,实现森林病虫害,尤其钻蛀性害虫的防控,是亟待攻克的难题之一。利用微生物介导的RNAi(MIGS, Microbe-induced gene silencing)将是重要突破口(Wen et al., 2023),也更适应于森林病虫害防治。
长喙壳类真菌已被证明易于在韧皮部、木质部、土壤和腐殖层中定殖,并在树木输导组织内传输(Jankowiak et al., 2021; Bilański et al., 2023)。此外,具备伸出基质延长的分生孢子梗和长颈的子囊壳和产囊体顶端聚生大量黏性分生孢子和子囊孢子,浓烈的芳香气味吸引媒介昆虫趋向。而且,其与小蠹虫等栖境相同等特点,使得长喙壳类真菌是一种理想的RNAi介导微生物,尤其具备良好的林间应用条件。
3.3 长喙壳类真菌野外增强小蠹虫引诱作用
长喙壳类真菌与小蠹虫的长期伴生使二者产生了趋同进化,即前者能够跨界产生小蠹虫的信息素(Zhao et al., 2015, 2019;Kandasamy et al., 2019)。室内试验表明,Grosmannia penicillata (Grosmann) Goid、E. polonica和Leptographium europhiides(E. F. Wright & Cain)M. Procter & Z. W. de Beer等产生的挥发性有机化合物能够成功吸引其媒介昆虫云杉八齿小蠹。野外试验发现,利用长喙壳类真菌能够实现林间增效、持效、长效诱捕云杉八齿小蠹(Jirošová et al., 2022)。并且除信息素外,真菌产生的杂醇和乙酸酯等混合物对小蠹虫同样具有吸引能力。这为利用长喙壳类真菌增强小蠹虫的引诱作用,实现对其的监测与防控提供了新思路。
3.4 应用长喙壳类真菌防治松材线虫病
长喙壳类真菌中的Esteya vermicola J. Y. Liou, J. Y. Shih & Tzean(Ev菌)已被证明对松材线虫具有杀灭效果,并且在防治实践中已被重点使用(Pires et al., 2022)。韩国利用Ev菌预先接种处理,可保护松树免受松材线虫侵染致死。松树存活率在处理后的5 a内(2009—2014年)一直保持在30%~50%,而未经Ev菌处理的对照,第1年即全部死亡。在6 a的试验期内,Ev菌都可以从接种的成年赤松中分离到(Wang et al., 2018)。近年来,在中国的云南切梢小蠹和华山松大小蠹伴生真菌中也分离获得了Ev菌(Wang et al., 2019, 2022)。该属的第二个种Esteya floridanum Y. Li, Araújo & Hulcr也展现出良好的预防松材线虫病效果(Li et al., 2021)。目前,在松材线虫病伴生真菌区系中,长喙壳类真菌群落也得到了越来越多的重视,其可能起到的拮抗松材线虫作用,是用于松材线虫病防治的良好生防资源(Vicente et al., 2022)。
-
表 1 长喙壳类真菌引起的主要病害
Table 1 Diseases caused by ophiostomatoid fungi
病原
Fungus分布
Distribution寄主
Host病害
Disease参考文献
ReferenceBretziella fagacearum 北美洲 栎树Quercus spp. 栎树枯萎病 de Beer et al., 2017 Ceratocystis acaciivora 南非 金合欢Acacia mearnsii 枯萎 Tarigan et al., 2011 C. albofundus 南非 金合欢A. mearnsii 枯萎
WiltRoux et al., 2007; Nasution et al., 2019 C. fimbriata 热带、亚热带和温带 多种农作物
Variety of agricultural crops腐烂、溃疡、枯萎 Baker et al., 2003 C. fimbriata 非洲中部、南部、
南美、亚洲桉树Eucalaptus spp. 枯萎 Roux et al., 2000; Roux and
Wingfield, 2009C. fimbriata f. platani 欧洲、北美洲 悬铃木Platanus spp. 溃疡、枯萎 Tsopelas and Angelopoulos, 2018 C. hulioh
C. lukuohia北美洲 铁心木
Metrosideros polymorpha枯萎 Barnes et al., 2018 C. manginecans 阿曼、巴基斯坦 芒果、金合欢
Mango, A. mearnsii枯萎 Van Wyk et al., 2007 C. pirilliformis 南非、澳大利亚 桉树Eucalaptus spp. 枯萎 Roux et al., 2004 C. tsitskammensis 南非 金合欢A. mearnsii 枯萎 Van der colff et al., 2017 Chalara australis 澳大利亚 假山毛榉Nothofagus spp. 枯萎 Kile and Walker, 1987 Davidsoniella virescens 北美洲 糖槭Acer sacharum 枯萎、腐烂 Bal et al., 2013 Endoconidiophora fujiensis 欧洲、日本、中国 落叶松Larix spp. 溃疡 Yamaoka et al., 1998; Liu et al., 2024 E. laricicola 欧洲、日本、中国 落叶松Larix spp. 溃疡 Yamaoka et al., 1998; 刘亚, 2024 E. polonica 欧洲、日本、中国 云杉Picea spp. 溃疡 Yamaoka et al., 2000;
Hlásny et al., 2021; 刘亚, 2024E. rufipenni 北美洲 云杉Picea spp. 溃疡 Solheim and Safranyik, 1997 Leptographium clavigerum 北美洲 美国黑松Pinus contorta 溃疡 DiGuistini et al., 2011 L. procerum 北美洲、欧洲、
新西兰、中国松树Pinus spp. 根病 Lu et al., 2009b L. qinlingense 中国 华山松P. armandii 溃疡 Wang et al., 2024a L. serpens 欧洲、南非 松树Pinus spp. 根病 Matusick et al., 2012 L. wageneri 北美洲 针叶树Conifers 黑根病 Cobb, 1988 L. wingfieldii 欧洲 松树Pinus spp. 溃疡 Hausner et al., 2005 L. yunnanense 中国 云南松P. yunnanensis 溃疡 Pan et al., 2018; 王慧敏, 2019 Ophiostoma dryocoetidis 北美洲 高山冷杉Abies lasiocarpa 溃疡 Lalande et al., 2020 O. minus 欧洲、北美洲 松树Pinus spp. 溃疡 Gorton and Webber, 2000 O. narcissus 北美洲、欧洲、新西兰 水仙花Narcissus spp. 黑根病 Davies et al., 1998 O. ulmi
O. novo-ulmi北美洲、欧洲、
亚洲、新西兰榆树Ulmus spp. 榆树枯萎病 Potter et al., 2011 Raffaelea lauricola 美国东南部 樟科Lauraceae 枯萎 Harrington et al., 2008;
Rodrigues et al., 2020Sporothrix schenkii
S. brasiliensis
S. globosa世界性的 人类Humans 孢子丝菌病 Rodrigues et al., 2013, 2020 Thielaviopsis paradoxa 热带、亚热带 多种农作物
Variety of agricultural crops根腐、果腐 Pinho et al., 2013 -
梁玲瑜, 王慧敏, 刘福, 等. 2024. 华山松切梢小蠹伴生真菌新种Geosmithia armandii及其对华山松的致病性[J]. 菌物学报, 43(6):17−31. Liang L Y, Wang H M, Liu F, et al. 2024. Geosmithia armandii, a new fungal species associated with Tmicus armandii and its pathogenicity to Pinus armandii[J]. Mycosystema, 43(6):17−31.
刘冰, 闫佳钰, 王朵, 等. 2024. 2023年全国主要林业有害生物发生情况及2024年趋势预测[J]. 中国森林病虫, 43(1): 41−45. Liu B, Yan J Y, Wang D, et al. 2024. Occurrence of major forest pests in China in 2023 and prediction for trend in 2024[J]. Forest Pest and Disease, 43(1): 41−45.
刘亚. 2024. 长喙壳真菌诱导寄主抗性的代谢组和转录组分析及 PkHGMR功能研究[D]. 北京: 中国林业科学研究院. Liu Y. 2024. Metabolomic and transcriptomica analyses of host resistance induced by ophiostematoid fungi and functional studies of PkHGMR[D]. Beijing: Chinese Academy of Forestry.
孟贤静, 吕全, 刘学伟, 等 .2015 . 落叶松八齿小蠹与长喙壳真菌间的种特异性伴生关系[J]. 生态学报,35 (2 ):313 −323 .Meng X J, Lyu Q, Liu X W, et al .2015 . Study on the species specific associations between Ips subelongatus and ophiostomatoid fungi[J]. Acta Ecologica Sinica,35 (2 ):313 −323 .田呈明, 张星耀. 2024. 树木医学的机遇与挑战[J]. 树木医学, 1(1): 1–8. Tian C M, Zhang X Y. 2024. Opportunities and challenges in tree medicine[J]. Tree Health, 1(1): 1–8.
王慧敏. 2019. 针叶林蛀干害虫伴生长喙壳类真菌多样性与致病性研究[D]. 北京: 中国林业科学研究院. Wang H M. 2019. Study on diversity and pathogenicity of ophiostomatoid fungi associated with stem-boring pests in coniferous forests[D]. Beijing: Chinese Academy of Forestry.
王正, 马晓乾, 周勤政, 等 .2021 . 中国齿小蠹属昆虫的鉴定[J]. 林业科学,57 (12 ):79 −91 .Wang Z, Ma X Q, Zhou Q Z, et al .2021 . Identification of Ips species (Coleoptera: Scolytinae) in China[J]. Scientia Silvae Sinicae,57 (12 ):79 −91 .张苏芳, 张荣, 张真 .2022 . 害虫种群分子调控技术及在森林害虫防控研究中的进展[J]. 中国森林病虫,41 (5 ):22 −31 .Zhang S F, Zhang R, Zhang Z .2022 . Molecular control technology of pest population and its research progress in forest pest control[J]. Forest Pest and Disease,41 (5 ):22 −31 .Alamouti S M, Wang V, Diguistini S, et al. 2011. Gene genealogies reveal cryptic species and host preferences for the pine fungal pathogen Grosmannia clavigera[J]. Molecular Ecology, 20(12): 2581–2602.
Ayres M P, Wilkens R T, Ruel J J .2000 . Nitrogen budgets of phloem-feeding bark beetles with and without symbiotic fungi[J]. Ecology,81 (8 ):2198 −2210 . DOI: 10.1890/0012-9658(2000)081[2198:NBOPFB]2.0.CO;2Baker C J, Harrington T C, Krauss U, et al .2003 . Genetic variability and host specialization in the Latin American clade of Ceratocystis fimbriata[J]. Phytopathology,93 (10 ):1274 −1284 . DOI: 10.1094/PHYTO.2003.93.10.1274Bal T L, Richter D, Storer A, et al. 2013. The relationship of the sapstreak fungus, Ceratocystis virescens, to sugar maple dieback and decay in northern Michigan[J]. American Journal of Plant Sciences, 4: 436-443.
Barnes I, Fourie A, Wingfield M J, et al. 2018. New Ceratocystis species associated with rapid death of Metrosideros polymorpha in Hawai’i[J]. Persoonia, 40(1): 154−181.
Bentz B J, Six D L. 2006. Ergosterol content of fungi associated with Dendroctonus ponderosae and Dendroctonus rufipennis (Coleoptera: Curculionidae, Scolytinae)[J]. Annals of the Entomological Society of America, 99(2): 189−194.
Bilański P, Jankowiak R, Solheim H, et al. 2023. Soil-borne Ophiostomatales species (Sordariomycetes, Ascomycota) in beech, oak, pine, and spruce stands in Poland with descriptions of Sporothrix roztoczensis sp. nov. , S. silvicola sp. nov. , and S. tumida sp. nov[J]. MycoKeys, 97: 41−69.
Bleiker K P, Six D L, 2014. Dietary benefits of fungal associates to an eruptive herbivore: potential implications of multiple associates on host population dynamics[J]. Environmental Entomology, 36(6): 1384–1396.
Bleiker K P, Six D L .2009 . Competition and coexistence in a multi-partner mutualism: interactions between two fungal symbionts of the mountain pine beetle in beetle-attacked trees[J]. Microbial Ecology,57 :191 −202 .Bracewell R R, Six D L. 2015. Experimental evidence of bark beetle adaptation to a fungal symbiont[J]. Ecology and Evolution, 5(21): 5109–5119.
Bracewell R R, Six D L. 2014. Broadscale specificity in a bark beetle-fungal symbiosis: a spatio-temporal analysis of the mycangial fungi of the western pine beetle[J]. Microbial Ecology, 68(4): 859–870.
Brasier C, Franceschini S, Forster J, et al .2021 . Enhanced outcrossing, directional selection and transgressive segregation drive evolution of novel phenotypes in hybrid swarms of the Dutch elm disease pathogen Ophiostoma novo-ulmi[J]. Journal of Fungi,7 (6 ):452 . DOI: 10.3390/jof7060452Cale J A, Ding R, Wang F, et al .2019 . Ophiostomatoid fungi can emit the bark beetle pheromone verbenone and other semiochemicals in media amended with various pine chemicals and beetle-released compounds[J]. Fungal Ecology,39 :285 −295 . DOI: 10.1016/j.funeco.2019.01.003Cale J A, Collignon R M, Klutsch J G, et al .2016 . Fungal volatiles can act as carbon sources and semiochemicals to mediate interspecific interactions among bark beetle-associated fungal symbionts[J]. PLoS One,11 (9 ):e0162197 . DOI: 10.1371/journal.pone.0162197Christiansen E, Waring R H, Berryman A A. 1987. Resistance of conifers to bark beetle attack: searching for general relationships[J]. Forest Ecology and Management, 22(1/2): 89−106.
Chang R, Duong T A, Taerum S J, et al. 2020. Ophiostomatoid fungi associated with mites phoretic on bark beetles in Qinghai, China[J]. IMA Fungus, 11: 15.
Chang R, Duong T A, Taerum S J, et al .2019 . Ophiostomatoid fungi associated with the spruce bark beetle Ips typographus, including 11 new species from China[J]. Persoonia,42 :50 −74 . DOI: 10.3767/persoonia.2019.42.03Cobb Jr F W. 1988. Leptographium wageneri, cause of black-stain root disease: a review of its discovery, occurrance and biology with emphasis on pinyon and ponderosa pine [M]. Cambridge: APS Press.
Cook S P, Shirley B M, Zambino P J .2010 . Nitrogen concentration in mountain pine beetle larvae reflects nitrogen status of the tree host and two fungal associates[J]. Environmental Entomology,39 (3 ):821 −826 . DOI: 10.1603/EN09305Davis T S. 2015. The ecology of yeasts in the bark beetle holobiont: a century of research revisited[J]. Microbial Ecology, 69(4): 723−732.
Davis T S, Landolt P J .2013 . A survey of insect assemblages responding to volatiles from a ubiquitous fungus in an agricultural landscape[J]. Journal of Chemical Ecology,39 (7 ):860 −868 .Davis T S, Stewart J E, Mann A, et al .2019 . Evidence for multiple ecological roles of Leptographium abietinum, a symbiotic fungus associated with the North American spruce beetle[J]. Fungal Ecology,38 :62 −70 .Davis T S, Crippen T L, Hofstetter R W T, et al. 2013. Microbial volatile emissions as insect semiochemicals[J]. Journal of Chemical Ecology, 39(7): 840−859.
Davis T S, Hofstetter R W, Foster J T, et al .2011 . Interactions between the yeast Ogataea pini and filamentous fungi associated with the western pine beetle[J]. Microbial Ecology,61 (3 ):626 −634 .Davies J M L, Dickens J S W, Inman A J, et al. 1998. Fungi associated with, and possible causes of, neck rot of Narcissus[J]. The Journal of Horticultural Science and Biotechnology, 73(2): 245−250.
de Beer Z W, Procter M, Wingfield M J, et al. 2022. Generic boundaries in the Ophiostomatales reconsidered and revised[J]. Studies in Mycology, 101: 57–120.
de Beer Z W, Marincowitz S, Duong T A, et al .2017 . Bretziella, a new genus to accommodate the oak wilt fungus, Ceratocystis fagacearum (Microascales, Ascomycota)[J]. MycoKeys,27 :1 −19 . DOI: 10.3897/mycokeys.27.20657de Beer Z W, Duong T A, Barnes I, et al .2014 . Redefining Ceratocystis and allied Genera[J]. Studies in Mycology,79 :187 −219 . DOI: 10.1016/j.simyco.2014.10.001de Beer Z W, Seifert K A, Wingfield M J. 2013. The ophiostomatoid fungi: their dual position in the Sordariomycetes[M]// Seifert K A, de Beer Z W, Wingfield M J (eds). The ophiostomatoid fungi: expanding frontiers. New York: Columbia Broadcasting System: 1–19.
DiGuistini S, Wang Y, Liao N Y, et al .2011 . Genome and transcriptome analyses of the mountain pine beetle fungal symbiont Grosmannia clavigera, a lodgepole pine pathogen[J]. Proceedings of the National Academy of Sciences of the United States of America,108 (6 ):2504 −2509 . DOI: 10.1073/pnas.1011289108Dowd P F. 1992. Insect fungal symbionts: A promising source of detoxifying enzymes[J]. Journal of Industrial Microbiology, 9(3): 149-161.
Fagan W F, Siemann E, Mitter C, et al. 2002. Nitrogen in insects: implications for trophic complexity and species diversification[J]. The American Naturalist, 160(6): 784−802.
Filipiak M, Weiner J .2014 . How to make a beetle out of wood: Multi-elemental stoichiometry of wood decay, xylophagy and fungivory[J]. PLoS One,9 (12 ):e115104 . DOI: 10.1371/journal.pone.0115104Flórez L V, Biedermann P H, Engl T W, et al. 2015. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms[J]. Natural Product Reports, 32(7): 904–936.
Franceschi V R, Krokene P, Christiansen E, et al. 2005. Anatomical and chemical defenses of conifer bark against bark beetles and other pests[J]. New Phytologist, 167(2): 353−375.
Giordano L, Garbelotto M, Nicolotti G, et al. 2013. Characterization of fungal communities associated with the bark beetle Ips typographus varies depending on detection method, location, and beetle population levels[J]. Mycological Progress, 12(1): 127–140.
Goodsman D W, Erbilgin N, Lieffers V J .2012 . The impact of phloem nutrients on overwintering mountain pine beetles and their fungal symbionts[J]. Environmental Entomology,41 (3 ):478 −486 . DOI: 10.1603/EN11205Gorton C, Webber J F .2000 . Reevaluation of the status of the bluestain fungus and bark beetle associate Ophiostoma minus[J]. Mycologia,92 (6 ):1071 −1079 . DOI: 10.1080/00275514.2000.12061254Grégoire J C, Evans H F. 2004. Damage and control of BAWBILT organisms an overview[M]. Lieutier F, Day K R, Battisti A, et al (eds). Bark and Wood Boring Insects in Living Trees in Europe, A Synthesis. Dordrecht: Kluwer Academic Publishers: 19–37.
Guerrero R, Margulis L, Berlanga M .2013 . Symbiogenesis: The holobiont as a unit of evolution[J]. International Microbiology,16 (3 ):133 −143 .Halsted B D .1890 . Some fungous diseases of the sweet potato[J]. New Jersey Agriculture Experiment Station Bulletin,76 :7 −14 .Hammerbacher A, Schmidt A, Wadke N, et al. 2013. A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce[J]. Plant Physiology, 162(3): 1324−1336.
Hausner G, Iranpour M, Kim J J, et al .2005 . Fungi vectored by the introduced bark beetle Tomicus piniperda in Ontario, Canada, and comments on the taxonomy of Leptographium lundbergii, Leptographium terebrantis, Leptographium truncatum, and Leptographium wingfieldii[J]. Botany,83 (10 ):1222 −1237 .Harrington T, Fraedrich S, Aghayeva D, 2008. Raffaelea lauricola, a new Ambrosia beetle symbiont and pathogen on the Lauracea[J]. Mycotaxon, 104: 399−404.
Harrington T C. 2005. Ecology and evolution of mycophagous bark beetles and their fungal partners[M]. In Insect-Fungal Associations: Ecology and Evolution. Oxford: Oxford University Press.
Harrington T C, Pashenova N V, McNew D L, et al. 2002. Species delimitation and host specialization of Ceratocystis laricicola and C. polonica to larch and spruce[J]. Plant Disease, 86(4): 418–422.
Hlásny T, Zimová S, Merganičová K, et al .2021 . Devastating outbreak of bark beetles in the Czech Republic: Drivers, impacts, and management implications[J]. Forest Ecology and Management,490 :119075 . DOI: 10.1016/j.foreco.2021.119075Hollowell H, Rieske L K .2022 . Southern pine beetle-specific RNA interference exhibits no effect on model nontarget insects[J]. Journal of Pest Science,95 (3 ):1429 −1441 . DOI: 10.1007/s10340-021-01473-1Hofstetter R W, Cronin J T, Klepzig K D, et al .2006 . Antagonisms, mutualisms and commensalisms affect outbreak dynamics of the southern pine beetle[J]. Oecologia,147 (4 ):679 −691 . DOI: 10.1007/s00442-005-0312-0Hofstetter R W, Mahfouz J B, Klepzig K D, et al. 2005. Effects of tree phytochemistry on the interactions among endophloedic fungi associated with the southern pine beetle[J]. Journal of Chemical Ecology, 31(3): 539−560.
Hulcr J, Dunn R R. 2011. The sudden emergence of pathogenicity in insect-fungus symbioses threatens naive forest ecosystems[J]. Proceedings. Biological Sciences, 278(1720): 2866−2873.
Itoh H, Tago K, Hayatsu M, et al. 2018. Detoxifying symbiosis: microbe-mediated detoxification of phytotoxins and pesticides in insects[J]. Natural Product Reports, 35(5): 434−454.
Jamil F, Mukhtar H, Fouillaud M, et al .2022 . Rhizosphere signaling: Insights into plant–rhizomicrobiome interactions for sustainable agronomy[J]. Microorganisms,10 (5 ):899 . DOI: 10.3390/microorganisms10050899Jankowiak R, Szewczyk G, Bilański P, et al .2021 . Blue‐stain fungi isolated from freshly felled Scots pine logs in Poland, including Leptographium sosnaicola sp. nov[J]. Forest Pathology,51 (2 ):e12672 . DOI: 10.1111/efp.12672Jirošová A, Modlinger R, Hradecký J, et al .2022 . Ophiostomatoid fungi synergize attraction of the Eurasian spruce bark beetle, Ips typographus to its aggregation pheromone in field traps[J]. Frontiers in Microbiology,13 :980251 . DOI: 10.3389/fmicb.2022.980251Kandasamy D, Gershenzon J, Andersson M N, et al .2019 . Volatile organic compounds influence the interaction of the Eurasian spruce bark beetle (Ips typographus) with its fungal symbionts[J]. The ISME Journal,13 (7 ):1788 −1800 . DOI: 10.1038/s41396-019-0390-3Keeling C I, Bohlmann J .2006 . Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens[J]. New Phytologist,170 (4 ):657 −675 . DOI: 10.1111/j.1469-8137.2006.01716.xKile G A, Walker J .1987 . Chalara australis sp. nov (Hyphomycetes), a vascular pathogen of Nothofagus cunninghamii (Fagaceae) in Australia and its relationship to other Chalara species[J]. Australian Journal of Botany,35 (1 ):1 −32 . DOI: 10.1071/BT9870001Kirisits T, 2004. Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi[M]. Lieutier F, Day K R, Battisti A, et al, eds. Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis. Dordrecht: Kluwer Academic Publishers: 181–235.
Klepzig K D. 1998. Competition between a biological control fungus, Ophiostoma piliferum, and symbionts of the southern pine beetle[J]. Mycologia, 90(1): 69–75.
Kolařík M, Hulcr J .2023 . Geosmithia—widespread and abundant but long ignored bark beetle symbionts[J]. Mycological Progress,22 (4 ):32 . DOI: 10.1007/s11557-023-01880-xKoski T M, Zhang B, Wickham J D, et al. 2024. Chemical interactions under the bark: bark-, ambrosia-, and wood-boring beetles and their microbial associates[J]. Reviews in Environmental Science and Bio/Technology, 23(4): 923–948.
Kostovcik M, Bateman C C, Kolarik M, et al. 2015. The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing[J]. The ISME Journal, 9(1): 126−138.
Krokene P, Solheim H, Långström B .2000 . Fungal infection and mechanical wounding induce disease resistance in Scots pine[J]. European Journal of Plant Pathology,106 (6 ):537 −541 . DOI: 10.1023/A:1008776002248Kyre B R, Bentz B J, Rieske L K .2020 . Susceptibility of mountain pine beetle (Dendroctonus ponderosae Hopkins) to gene silencing through RNAi provides potential as a novel management tool[J]. Forest Ecology and Management,473 :118322 . DOI: 10.1016/j.foreco.2020.118322Kyre B R, Rodrigues T B, Rieske L K .2019 . RNA interference and validation of reference genes for gene expression analyses using qPCR in southern pine beetle, Dendroctonus frontalis[J]. Scientific Reports,9 (1 ):5640 . DOI: 10.1038/s41598-019-42072-6Lalande B M, Hughes K, Jacobi W R, et al .2020 . Subalpine fir mortality in Colorado is associated with stand density, warming climates and interactions among fungal diseases and the western balsam bark beetle[J]. Forest Ecology and Management,466 :118133 . DOI: 10.1016/j.foreco.2020.118133Lee D H, Nam Y, Wingfield M J. et al .2022 . First report of Dutch elm disease caused by Ophiostoma novo-ulmi in south Korea[J]. Forests,13 (7 ):968 . DOI: 10.3390/f13070968Lee S, Hamelin R C, Six D L. et al. 2007. Genetic diversity and the presence of two distinct groups in Ophiostoma clavigerum associated with Dendroctonus ponderosae in British Columbia and the northern rocky mountains[J]. Phytopathology, 97(9): 1177–1185.
Li Y, Yu H Y, Araújo J P M, et al .2021 . Esteya floridanum sp. nov: An ophiostomatalean nematophagous fungus and its potential to control the pine wood nematode[J]. Phytopathology,111 (2 ):304 −311 .Lieutier F, Yart A, Salle A, 2009. Stimulation of tree defenses by Ophiostomatoid fungi can explain attack success of bark beetles on conifers[J]. Annals of Forest Science, 66(8): 801.
Lieutier F, Sauvard D, Brignolas F, et al. 1996. Changes in phenolic metabolites of Scots‐pine phloem induced by Ophiostoma brunneo‐ciliatum, a bark‐beetle‐associated fungus[J]. European Journal of Forest Pathology, 26(3): 145−158.
Lieutier F, Vouland G, Pettinetti M, et al. 1992. Defence reactions of Norway spruce (Picea abies Karst. ) to artificial insertion of Dendroctonus micans Kug. (Col. , Scolytidae).[J]. Journal of Applied Entomology, 114(1–5): 174–186.
Lim W Y, Kim J J, Lu M, et al. 2005. Determining fungal diversity on Dendroctonus ponderosae and Ips pini affecting lodgepole pine using cultural and molecular methods[J]. Fungal Divers, 19(9): 79–94.
Liu Y, Zhou Q Z, Wu D, et al. 2024. Pathogenicity and induced resistance in Larix kaempferi and Larix olgensis inoculated with Endoconidiophora fujiensis.[J]. Tree Physiology, 44(7): tpae069.
Liu Y, Zhou Q Z, Wang Z, et al. 2022a. Pathophysiology and transcriptomic analysis of Picea koraiensis inoculated by bark beetle-vectored fungus Ophiostoma bicolor[J]. Frontiers in Plant Science, 13: 944336.
Liu F H, Ye F Y, Cheng C, et al. 2022b. Symbiotic microbes aid host adaptation by metabolizing a deterrent host pine carbohydrate d-pinitol in a beetle-fungus invasive complex[J]. Science Advances, 8(51): eadd5051.
Liu Y Z, Anastacio G R, Ishangulyyeva G, et al. 2021. Mutualistic Ophiostomatoid fungi equally benefit from both a bark beetle pheromone and host tree volatiles as nutrient sources[J]. Microbial Ecology, 81(4): 1106–1110.
Liu F H, Wickham J D, Cao Q J, et al .2020 . An invasive beetle–fungus complex is maintained by fungal nutritional-compensation mediated by bacterial volatiles[J]. The ISME Journal,14 (11 ):2829 −2842 . DOI: 10.1038/s41396-020-00740-wLu M, Wingfield M J, Gillette N, et al .2011 . Do novel genotypes drive the success of an invasive bark beetle-fungus complex? Implications for potential reinvasion[J]. Ecology,92 (11 ):2013 −2019 . DOI: 10.1890/11-0687.1Lu M, Wingfield M J, Gillette N E, et al .2010 . Complex interactions among host pines and fungi vectored by an invasive bark beetle[J]. New Phytologist,187 (3 ):859 −866 . DOI: 10.1111/j.1469-8137.2010.03316.xLu M, Zhou X D, De Beer Z W, et al. 2009a. Ophiostomatoid fungi associated with the invasive pine-infesting bark beetle, Dendroctonus valens, in China[J]. Fungal Divers, 38: 133–145.
Lu Q, Decock C, Zhang X Y, et al. 2009b. Ophiostomatoid fungi (Ascomycota) associated with Pinus tabuliformis infested by Dendroctonus valens (Coleoptera) in Northern China and an assessment of their pathogenicity on mature trees[J]. Antonie Van Leeuwenhoek, 96(3): 275–293.
Macias-Rodriguez L, Contreras-Cornejo H, Lopez-Bucio J, et al. 2015. Chapter 7: recent advancements in the role of volatile organic compounds from fungi[M]. //Gupta V, Mach R, Sreenivasaprasad S. (Eds). Fungal Biomolecules: Sources, Applications and Recent Developments. John Wiley & Sons, Ltd, West Sussex, UK, 87–99.
Marin M, Preisig O, Wingfield B D, et al. 2009. Single sequence repeat markers reflect diversity and geographic barriers in Eurasian populations of the conifer pathogen Ceratocystis polonica[J]. Forest Pathology, 39(4): 249–265.
Marin M, Preisig O, Wingfield B D, et al. 2005. Phenotypic and DNA sequence data comparisons reveal three discrete species in the Ceratocystis polonica species complex[J]. Mycological Research, 109(10): 1137–1148.
Marincowitz S, Duong T A, Taerum S J, et al. 2020. Fungal associates of an invasive pine-infesting bark beetle, Dendroctonus valens, including seven new Ophiostomatalean fungi[J]. Persoonia, 45: 177–195.
Masuya H, Brasier C, Ichihara Y, et al .2010 . First report of the Dutch elm disease pathogens Ophiostoma ulmi and O. novo-ulmi in Japan[J]. Plant Pathology,59 (4 ):805 .Matusick G, Somers G L, Eckhardt L G .2012 . Root lesions in large loblolly pine (Pinus taeda L. ) following inoculation with four root‐inhabiting ophiostomatoid fungi[J]. Forest Pathology,42 (1 ):37 −43 . DOI: 10.1111/j.1439-0329.2011.00719.xMayer F, Piel F B, Cassel-Lundhagen A, et al. 2015. Comparative multilocus phylogeography of two palaearctic spruce bark beetles: influence of contrasting ecological strategies on genetic variation[J]. Molecular Ecology, 24(6): 1292–1310.
McLeod G, Gries R, Von Reuss S H, et al .2005 . The pathogen causing Dutch elm disease makes host trees attract insect vectors[J]. Proceedings. Biological Sciences,272 (1580 ):2499 −2503 . DOI: 10.1098/rspb.2005.3202Miyamoto T, Masuya H, Koizumi A, et al .2019 . A report of dieback and mortality of elm trees suspected of Dutch elm disease in Hokkaido, Japan[J]. Journal of Forest Research,24 (6 ):396 −400 . DOI: 10.1080/13416979.2019.1679942Morath S U, Hung R, Bennett J W. 2012. Fungal volatile organic compounds: a review with emphasis on their biotechnological potential[J]. Biology Reviews, 26(2−3): 73−83.
Nasution A, Glen M, Beadle C, et al .2019 . Ceratocystis wilt and canker–a disease that compromises the growing of commercial Acacia-based plantations in the tropics[J]. Australian Forestry,82 (sup1 ):80 −93 . DOI: 10.1080/00049158.2019.1595347Paciura D, de Beer Z W, Jacobs K, et al. 2010. Eight new Leptographium species associated with tree-infesting bark beetles in China[J]. Persoonia, 25: 94–108.
Pan Y, Zhao T, Krokene P, et al .2018 . Bark beetle-associated blue-stain fungi increase antioxidant enzyme activities and monoterpene concentrations in Pinus yunnanensis[J]. Frontiers in Plant Science,9 :1731 . DOI: 10.3389/fpls.2018.01731Pepori A L, Bettini P P, Comparini C, et al .2018 . Geosmithia-Ophiostoma: A new fungus-fungus association[J]. Microbial Ecology,75 (3 ):632 −646 . DOI: 10.1007/s00248-017-1062-3Pinho D B, Dutra D C, Pereira O L .2013 . Notes on Ceratocystis paradoxa causing internal post-harvest rot disease on immature coconut in Brazil[J]. Tropical Plant Pathology,38 (2 ):152 −157 . DOI: 10.1590/S1982-56762013000200010Pires D, Vicente C S L, Inácio M L, et al .2022 . The potential of Esteya spp. for the biocontrol of the pinewood pematode, Bursaphelenchus xylophilus[J]. Microorganisms,10 (1 ):168 . DOI: 10.3390/microorganisms10010168Ploetz R C. 2003. Diseases of tropical fruits crops[M]. Wallingford: CABI Publishing.
Popkin G. 2021. Forest fight[J]. Science, 374(6572): 1184–1189.
Postma J, Goossen-van de Geijn H .2016 . Twenty-four years of Dutch Trig® application to control Dutch elm disease[J]. BioControl,61 (3 ):305 −312 . DOI: 10.1007/s10526-016-9731-6Potter C, Harwood T, Knight J, et al. 2011. Learning from history, predicting the future: The UK Dutch elm disease outbreak in relation to contemporary tree disease threats[J]. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 366(1573): 1966−1974.
Ranger C M, Biedermann P H W, Phuntumart V, et al. 2018. Symbiont selection via alcohol benefits fungus farming by Ambrosia beetles[J]. Proceedings of the National Academy of Sciences of the United States of America, 115(17): 4447−4452.
Raffa K F, Gregoire J C, Lindgren B S. 2015. Natural history and ecology of bark beetles[M]// Vega FE, W. HR (eds) Bark Beetles: Biology and Ecology of Native and Invasive Species. San Diego: Elsevier: 1–40.
Rigling D, Prospero S .2018 . Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control[J]. Molecular Plant Pathology,19 (1 ):7 −20 . DOI: 10.1111/mpp.12542Rodrigues A M, de Hoog S, de Camargo Z P .2013 . Emergence of pathogenicity in the Sporothrix schenckii complex[J]. Medical Mycology,51 (4 ):405 −412 . DOI: 10.3109/13693786.2012.719648Rodrigues A M, Della Terra P P, de Gremião I D, et al .2020 . The threat of emerging and re-emerging pathogenic Sporothrix species[J]. Mycopathologia,185 (5 ):813 −842 . DOI: 10.1007/s11046-020-00425-0Roe A D, Rice A V, Coltman D W, et al. 2011. Comparative phylogeography, genetic differentiation and contrasting reproductive modes in three fungal symbionts of a multipartite bark beetle symbiosis[J]. Molecular Ecology, 20(3): 584–600.
Roux J, Wingfield M J. 2009. Ceratocystis species: Emerging pathogens of non-native plantation Eucalyptus and Acacia species[J]. Southern Forests: a Journal of Forest Science, 71(2): 115–120.
Roux J, Heath R N, Labuschagne L, et al .2007 . Occurrence of the wattle wilt pathogen, Ceratocystis albifundus on native South African trees[J]. Forest Pathology,37 (5 ):292 −302 . DOI: 10.1111/j.1439-0329.2007.00507.xRoux J V, Van Wyk M, Hatting H, et al .2004 . Ceratocystis species infecting stem wounds on Eucalyptus grandis in South Africa[J]. Plant Pathology,53 (4 ):414 −421 . DOI: 10.1111/j.0032-0862.2004.01014.xRoux J, Wingfield M J, Bouillet J P, et al .2000 . A serious new wilt disease of Eucalyptus caused by Ceratocystis fimbriata in Central Africa[J]. Forest Pathology,30 (3 ):175 −184 . DOI: 10.1046/j.1439-0329.2000.00202.xSallé A, Arthofer W, Lieutier F, et al. 2007. Phylogeography of a host-specific insect: Genetic structure of Ips typographus in Europe does not reflect past fragmentation of its host[J]. Biological Journal of the Linnean Society, 90(2): 239–246.
Schulz-Bohm K, Martín-Sánchez L, Garbeva P, 2017. Microbial volatiles: Small molecules with an important role in intra- and inter-Kingdom interactions[J]. Frontiers in Microbiology, 8: 2484.
Scriber J M, Slansky F Jr .1981 . The nutritional ecology of immature insects[J]. Annual Review of Entomology,26 :183 −211 . DOI: 10.1146/annurev.en.26.010181.001151Shi Z H, Wang B, Clarke S R, et al .2012 . Effect of associated fungi on the immunocompetence of red turpentine beetle larvae, Dendroctonus valens (Coleoptera: Curculionidae: Scolytinae)[J]. Insect Science,19 (5 ):579 −584 . DOI: 10.1111/j.1744-7917.2011.01484.xSingewar K, Fladung M .2023 . Double-stranded RNA (dsRNA) technology to control forest insect pests and fungal pathogens: Challenges and opportunities[J]. Functional & Integrative Genomics,23 (2 ):185 .Six D L. 2012. Ecological and evolutionary determinants of bark beetle-fungus symbioses[J]. Insects, 3(1): 339–366.
Six D L, Paine T D. 1997. Ophiostoma clavigerum is the mycangial fungus of the Jeffrey pine beetle, Dendroctonus jeffreyi[J]. Mycologia, 89(6): 858–866.
Solheim H, Safranyik L .1997 . Pathogenicity to Sitka spruce of Ceratocystis rufipenni and Leptographium abietinum blue-stain fungi associated with the spruce beetle[J]. Canadian Journal of Forest Research,27 (9 ):1336 −1341 . DOI: 10.1139/x97-096Solheim H .1993 . Fungi associated with the spruce bark beetle Ips typographus in an endemic area in Norway[J]. Scandinavian Journal of Forest Research,8 (1–4 ):118 −122 . DOI: 10.1080/02827589309382760Solheim H .1992 . Fungal succession in sapwood of Norway spruce infested by the bark beetle Ips typographus[J]. European Journal of Forest Pathology,22 (3 ):136 −148 . DOI: 10.1111/j.1439-0329.1992.tb01440.xSong L W, Zhang Q H, Chen Y Q, et al. 2011. Field responses of the Asian larch bark beetle, Ips subelongatus, to potential aggregation pheromone components: Disparity between two populations in Northeastern China[J]. Insect Science, 18(3): 311–319.
Stauffer C, Lakatos F. 2000. Ips typographus f. japonicus Niijima (Col. , Scolytidae): a genetic analysis by allozymes and mitochondrial sequence data[M]//Forests and Society: The Role of Research. XXI IUFRO World Congress, Malaysia: Pramaju Sdn.
Tarigan M, Roux J, Van Wyk M. et al. 2011. A new wilt and die-back disease of Acacia mangium associated with Ceratocystis manginecans and C. acaciivora sp. nov. in Indonesia[J]. South African Journal of Botany, 77(2): 292–304.
Tsopelas P, Angelopoulos A, 2018. First report of canker stain disease of plane trees, caused by Ceratocystis fimbriata f. sp. platani in Greece [J]. Plant Pathology, 53(4): 531.
Van der Colff D, Dreyer L L, Valentine A, et al .2017 . Differences in physiological responses to infection by Ceratocystis tsitsikammensis, a native ophiostomatoid pathogen, between a native forest and an exotic forestry tree in South Africa[J]. Fungal Ecology,27 :107 −115 . DOI: 10.1016/j.funeco.2016.06.003Van Wyk M, Adawi A O, Khan I A, et al. 2007. Ceratocystis manginecans sp. nov. , causal agent of a destructive mango wilt disease in Oman and Pakistan[J]. Fungal Divers, 27: 213–230.
Vicente C S L, Soares M, Faria J M S, et al. 2022. Fungal communities of the pine wilt disease complex: studying the interaction of ophiostomatales with Bursaphelenchus xylophilus[J]. Frontiers in Plant Science, 13: 908308.
Wadke N, Kandasamy D, Vogel H, et al. 2016. The bark-beetle-associated fungus, Endoconidiophora polonica, utilizes the phenolic defense compounds of its host as a carbon source[J]. Plant Physiology, 171(2): 914−931.
Wallin K F, Raffa K F .2000 . Influences of host chemicals and internal physiology on the multiple steps of postlanding host acceptance behavior of Ips pini (Coleoptera: Scolytidae)[J]. Environmental Entomology,29 (3 ):442 −453 . DOI: 10.1603/0046-225X-29.3.442Wang C Y, Yin C, Fang Z M, et al, 2018. Using the nematophagous fungus Esteya vermicola to control the disastrous pine wilt disease[J]. Biocontrol Science and Technology, 28(3): 268-277.
Wang H M, Liu F, Zhang S F, et al. 2019. Epibiotic fungal communities ofthree Tomicus spp. infesting pines in south Western China[J]. Microorganisms, 8(1): 15.
Wang H M, Liu Y, Wang T T, et al .2024a . Pathophysiology and transcriptomic responses of Pinus armandii defenses to ophiostomatoid fungi[J]. Tree Physiology,44 (6 ):tpae056 . DOI: 10.1093/treephys/tpae056Wang H M, Wang T T, Liu Y, et al. 2022. Diversity of ophiostomatoid fungiassociated with dendroctonus armandi infesting Pinus armandii inWestern China[J]. Journal of Fungi, 8(3): 214.
Wang Z, Liang L Y, Wang H M, et al. 2024b. Ophiostomatoid fungi associated with Ips bark beetles in China [J]. Fungal Diversity, 129(1): 283−364.
Wang Z, Liu C X, Song X Y, et al .2024c . Ophiostomatalean fungi associated with Polygraphus bark beetles in the Qinghai-Tibet Plateau, China[J]. MycoKeys,110 :93 −115 . DOI: 10.3897/mycokeys.110.135538Wang Z, Liu Y, Wang H M, et al. 2023. Genome and transcriptome of Ips nitidus provide insights into high-altitude hypoxia adaptation and symbiosis[J]. iScience, 26(10): 107793.
Wang Z, Zhou Q Z, Zheng G H, et al. 2021. Abundance and diversity of ophiostomatoid fungi associated with the great spruce bark beetle (Dendroctonus micans) in the northeastern Qinghai-Tibet Plateau[J]. Frontiers in Microbiology, 12: 721395.
Wang Z, Liu Y, Wang H, et al. 2020. Ophiostomatoid fungi associated with Ips subelongatus, including eight new species from Northeastern China[J]. IMA Fungus, 11: 3.
Wen H G, Zhao J H, Zhang B S, et al. 2023. Microbe-induced gene silencing boosts crop protection against soil-borne fungal pathogenss[J]. Nature Plants, 9(9): 1409–1418.
Williams G M, Ginzel M D, Ma Z, et al .2023 . The global forest health crisis: A public-good social dilemma in need of international collective action[J]. Annual Review of Phytopathology,61 (1 ):377 −401 . DOI: 10.1146/annurev-phyto-021722-024626Wingfield M J, Seifert K, Webber J F. 1993. Ceratocystis and Ophiostoma: taxonomy, ecology, and pathogenicity[M]. Minnesota: American Phytopathological Society Press.
Yamaoka Y, Takahashi I, Iguchi K .2000 . Virulence of ophiostomatoid fungi associated with the spruce bark beetle Ips typographus f. japonicus in Yezo spruce[J]. Journal of Forest Research,5 (2 ):87 −94 . DOI: 10.1007/BF02762525Yamaoka Y L, Wingfield M J, Ohsawa M D, et al .1998 . Ophiostomatoid fungi associated with Ips cembrae in Japan and their pathogenicity to Japanese larch[J]. Mycoscience,39 (4 ):367 −378 .Yin M, Wingfield M J, Zhou X, et al. 2016. Multigene phylogenies and morphological characterization of five new Ophiostoma spp. associated with spruce-infesting bark beetles in China[J]. Fungal Biology, 120(4): 454–470.
Zhao T, Ganji S, Schiebe C, et al .2019 . Convergent evolution of semiochemicals across Kingdoms: Bark beetles and their fungal symbionts[J]. The ISME Journal,13 (6 ):1535 −1545 . DOI: 10.1038/s41396-019-0370-7Zhao T, Axelsson K, Krokene P, et al. 2015. Fungal symbionts of the spruce bark beetle synthesize the beetle aggregation pheromone 2-methyl-3-buten-2-ol[J]. Journal of Chemical Ecology, 41(9): 848–852.
Zhou F Y, Xu L T, Wang S S, et al. 2017. Bacterial volatile ammonia regulates the consumption sequence of d-pinitol and d-glucose in a fungus associated with an invasive bark beetle[J]. The ISME Journal, 11(12): 2809–2820.
Zhou X D, Jacobs K, Morelet M, et al. 2000. A new Leptographium species associated with Tomicus piniperda in south-western China[J]. Mycoscience, 41(6) : 573–578.
Zhu Y Y, Lu A N, Wang Z, et al. 2022. Molecular characterization of a novel endornavirus isolated from Ophiostoma bicolor associated with bark beetles[J]. Archives of Virology, 167(12): 2839–2843.



 下载:
下载: